Healthier Populations, Everywhere
Filters
Region
Country
Focus Areas
Vaccine Preventable Diseases
Client

Evaluating the impact of electronic Logistic Management Information Systems (eLMIS) and electronic Immunisation Registries (EIR) in low- and middle-income countries
Multi-country evaluation generating evidence on the effectiveness, affordability and sustainability of eIR and eLMIS in low- and middle-income countries.
Bill & Melinda Gates Foundation, Gavi, The Vaccine Alliance (Gavi)

Coalition for Epidemic Preparedness Innovation (CEPI) mid-term review
Conduct a mid-term review of CEPI to help improve operations and vaccine development.
Coalition for Epidemic Preparedness Innovation (CEPI)

Progress report on the European Agenda 2030 (EIA2030)
Support the WHO European Regional Office to monitor progress on the implementation of the European Immunization Agenda 2030 (EIA2030).
World Health Organisation (WHO)

Assess the UN supply chain system COVID-19 response
Identifying areas for improving pandemic readiness in the UN supply chain for biomedical supplies.
World Health Organisation (WHO)

Evaluating the INCLEN Trust Childhood Pneumonia Programme in India
Independent evaluation of a childhood pneumonia research project.
The INCLEN Trust International

Evaluating tier 2 surveillance sites for the National Surveillance System for Enteric Fever in India
An independent site evaluation of a multicentric surveillance project for enteric fever in India.
Christian Medical College

Gap analysis of evidence and information on the acceptability and access to maternal immunization
Gap analysis of evidence and information on the acceptability of, and access to, maternal immunization that contributed to the development of a roadmap for advancing maternal immunization against Respiratory Syncytial Virus (RSV).
ISGlobal

Mid-term evaluation of the European Vaccine Action Plan 2015-2020
Mid-term progress report of the European Vaccine Action Plan 2015-2020.
World Health Organisation (WHO)

Evaluating WHO’s role in supporting early COVID-19 sero-epidemiologic studies: the Unity Initiative
MMGH is evaluating WHO Health Emergency Program’s role in supporting countries to conduct COVID-19 sero-epidemiologic investigations, the “Unity initiative”. This initiative developed scientifically vetted and standardised protocols to facilitate the conduct of early serologic studies in any resources setting. The purpose of these studies is to inform country actions towards protecting their vulnerable populations and citisens at large. MMGH is gathering and synthesizing information on how countries used UNITY resources (study protocols, technical assistance, financial support) and how WHO and partners managed and facilitated support. The aim is to document what worked well and what can be optimised in terms of readiness to support high-quality investigations in future large-scale outbreaks.
World Health Organisation (WHO)

COVID-19 vaccine post-introduction evaluations (cPIEs) in low- and middle-income countries
MMGH is supporting the Task Force for Global Health, US CDC and WHO in conducting cPIEs in 8 countries. This includes the development of generic training materials for the online and/or face-to-face training of evaluators and the actual conduct of the respective training sessions. MMGH staff will lead cPIEs in three countries, which includes preparation and implementation of the evaluation, the synthesis of findings, the debriefing and report writing. MMGH staff will also participate in 5 additional cPIEs and will summarise cross-learnings to share with other countries.
Task Force for Global Health

Evaluation of linking supplementary immunisation activities and routine immunisation in Nepal
MMGH assisted the WHO Country Office and Ministry of Health (MoH) Nepal in the evaluation of a new initiative linking supplementary immunisation activities (SIAs), against measles and rubella, with intensified follow-up of children who had missed their routine immunisation doses. MMGH collaborated with Routes 2 Results in designing a mixed methods research protocol, developing and pre-testing survey and data collection forms, and supervising a local agency carrying out the research in four provinces of the country. The work will inform the MoH of Nepal and of other countries about the effectiveness of the new interventions to strengthen routine immunisation service delivery through an SIA-based approach.
World Health Organisation (WHO)

Evaluation of the Global Vaccine Action Plan 2011-2020
MMGH supported the WHO Department of Immunisation, Vaccines and Biologicals (IVB) in the evaluation of strengths and weaknesses of the current Global Vaccine Action Plan (GVAP), its monitoring and evaluation and accountability framework, and the added value it provides to the global immunisation arena. The project included a comparative landscape analysis of global immunisation in 2010 and 2018, and a description of the overall progress on the plan’s goals and strategic objectives. In this project, MMGH partnered with the Task Force for Global Health.
World Health Organisation (WHO)

Evaluation of WHO’s Strategic Advisory Group of Experts on Immunisation (SAGE)
MMGH supported the WHO Department of Immunisation, Vaccines and Biologicals (IVB) and the Expert Advisory Group on SAGE Evaluation (EAGSE) in assessing the relevance, effectiveness and quality of SAGE’s processes and deliverables. The evaluation was to ensure that SAGE continues to provide high quality strategic advice in all areas of the evolving immunisation and global health agenda. The project included scoping the evaluation, performing stakeholder consultations, gathering and analysing findings, facilitating the review by the EAGSE, and developing recommendations and the evaluation report.
World Health Organisation (WHO)
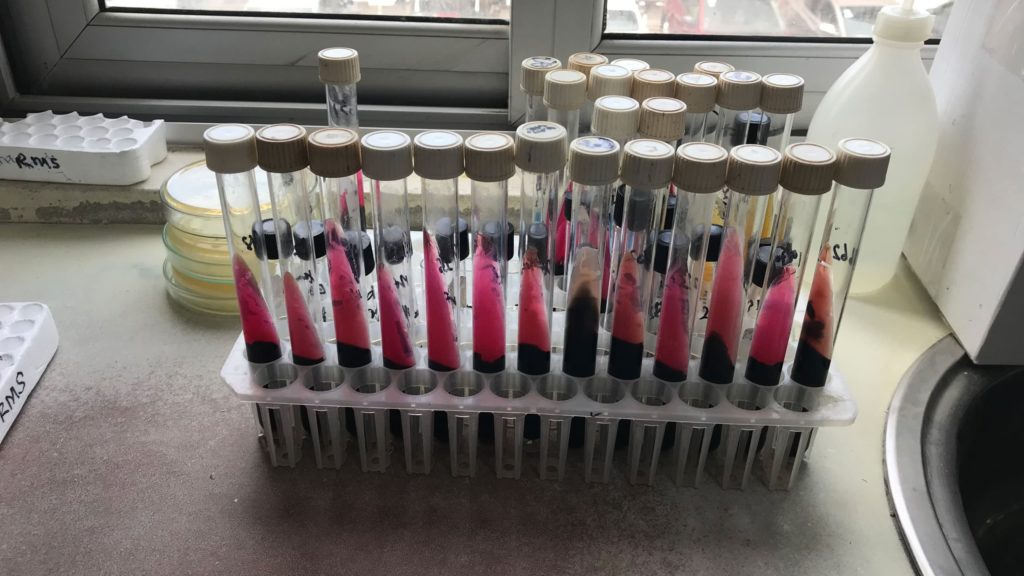
Evaluation of the enteric fever surveillance pilot programme in Ghana
MMGH supported the WHO Initiative for Vaccine Research (IVR) in assessing the feasibility and reliability of integrating surveillance of enteric fever (EF) and invasive non-typhoidal salmonellosis (iNTS) in the WHO-coordinated Global Invasive Bacterial Vaccine Preventable Disease Surveillance Network. In Ghana, MMGH conducted two successive evaluations of the EF and iNTS surveillance pilot programmes using a newly developed structured assessment tool. The evaluation included on-site reviews of clinical and epidemiological aspects of the surveillance system, the assessment of the participating microbiology laboratories, and of overall data quality and surveillance network management.
World Health Organisation (WHO)

Mid-Term review of the Regional Strategic Plan for Immunisation for the African Region
MMGH supported the WHO Regional Office for Africa (AFRO) in performing the mid-term review of the Regional Strategic Plan for Immunisation 2014-2020. MMGH prepared an extensive desk review, facilitated a face-to-face session of the external review panel, summarised and consolidated review findings, assisted in the development of recommendations and in the presentation of a final evaluation report to the AFRO Regional Immunisation Technical Advisory Group.
World Health Organisation (WHO)

Review of Global Vaccine Action Plan 2011-2020
MMGH supported the WHO Global Vaccine Action Plan (GVAP) Secretariat in developing a process and structure of the GVAP follow-up plan for the period 2021 – 2030. MMGH performed in-depth consultations with key stakeholders, presented summary findings and a suggested way forward to the WHO Strategic Advisory Group of Experts (SAGE) in Immunisation, including the development of a timeline and plan of operations for submission of the new GVAP to the 2020 World Health Assembly.
World Health Organisation (WHO)
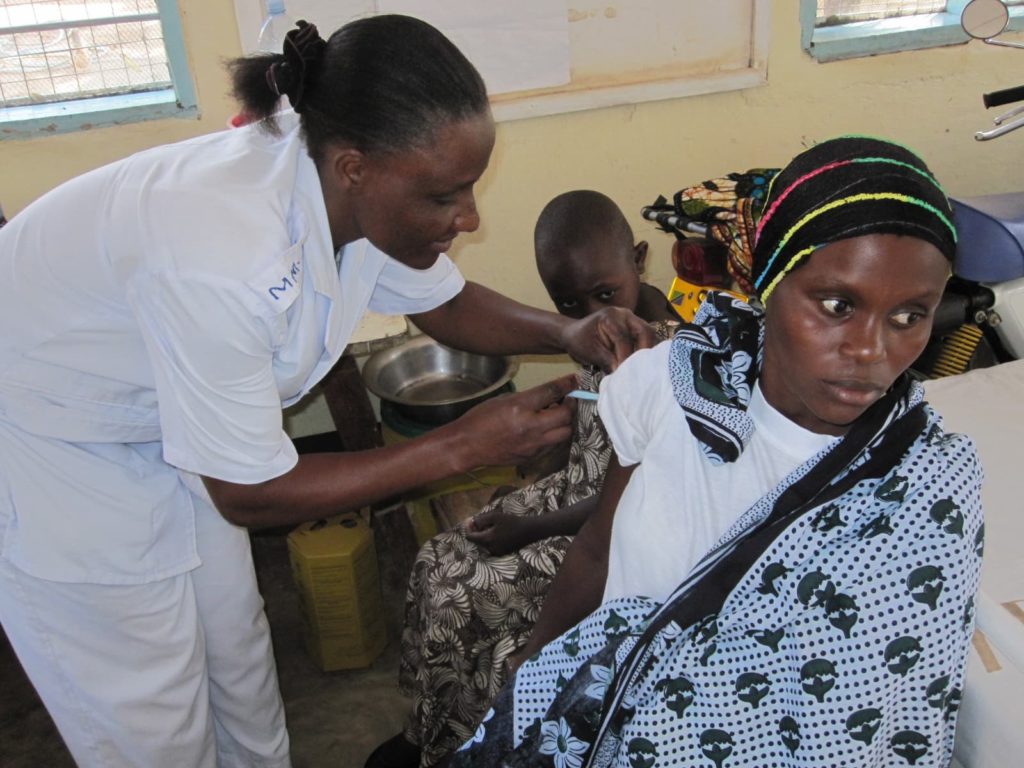
Review of Gavi-supported data strengthening activities
MMGH supported the Monitoring & Evaluation Unit of the Gavi Secretariat in performing a review and synthesis of Gavi-funded data strengthening partner activities of WHO, UNICEF, US CDC, and WB and presented findings at a Monitoring, Data Systems and Strategic Information partner workshop which defined the future direction of this work.
Gavi, The Vaccine Alliance (Gavi)

Documentation of lessons learnt from the Oral Cholera Vaccine campaign in Haiti
MMGH supported the WHO Infectious Hazard Management (IHM) team in performing an evaluation of the management processes of the Oral Cholera Vaccine (OCV) campaign in Haiti. MMGH conducted interviews with key stakeholders and summarised the findings to support the Global Task Force on Cholera Control (GTFCC) processes re-engineering discussion.
World Health Organisation (WHO)

Review of the Oral Cholera Vaccine Working Group of the Global Task Force on Cholera Control
MMGH supported the WHO in assessing the functioning of the Oral Cholera Vaccine (OCV) Working Group of the Global Task Force on Cholera Control (GTFCC). MMGH performed stakeholder interviews and prepared a White Paper that summarised successes and areas for improvement contributing to the redesign of the WG processes.
World Health Organisation (WHO)
